Banned gases reveal the age of water
The use of gases that deplete the ozone layer has been restricted for almost forty years. Still such substances linger in the ocean – a troublesome legacy marine scientists can exploit to keep track of the ocean circulation.
Publisert 03. November 2025
Written by Ellen Viste

Though freons and other CFCs are being phased out, such substances still linger in the ocean and the atmosphere. Photo: Ellen Viste
For more than fifty years CFC gases like freons and halons were used in everything from refrigerators and fire extinguishing systems to insulation foams and hairspray. Stable and of low toxicity, these gases were well-suited for so many applications that the CFC concentration in the atmosphere increased exponentially from the 1960s.
Then it was discovered that CFCs harmed the ozone layer. In 1987 46 nations signed the Montreal Protocol, agreeing to phase out the use of CFCs. By now all the world's nations have signed the protocol, an example of a successful international environmental agreement.
Since around the millennium the concentration of CFCs in the atmosphere has declined, and the ozone layer has begun to recover. But, as CFCs break down slowly, a large share of the emitted substances remains in nature.
Something good still comes out of this. Only trace amounts of CFCs in sea water are required to detect their presence. Such measurements may be used to follow the water's journey into the depths – crucial for monitoring changes in ocean currents.
CFC
- Chlorofluorocarbons (CFCs) are hydrocarbons in which one or more hydrogen atoms have been replaced by chlorine or fluorine, or in some cases bromine.
- CFCs are very stable and of low toxicity.
- CFCs break down in the stratosphere, freeing chlorine atoms that cause the depletion of ozone.
- CFCs also contribute to increasing the greenhouse effect.
- The half-lives of CFCs in the atmosphere are between 55 and 140 years, implying that it takes a long time to break down the gases already emitted.
Source: snl.no

Sea water has been brought onboard, and Emil Jeansson collects samples for analysis in the laboratory. Photo: Ellen Viste
Sea water sinks to great depths
"Here we find the age of the water."
Emil Jeansson points to a sturdy steel frame secured to a bench with a cargo strap. The oceanographer from the Bjerknes Centre is the Research Director of Ocean Observations at NORCE.
The bench with the steel framed device stands in a laboratory onboard the research vessel Kronprins Haakon, on a cruise in the Greenland Sea.

In case of rough seas, all instruments in the ship's laboratory are strapped to the bench. The device to the left was built at the Bjerknes Centre in 2008 and has been used regularly on research cruises. It extracts CFCs and other substances that the scientists want to analyze. Afterward the amount of these substances is registered in the white box next to it, a gas chromatograph. The results appear on the computer screen to the right. Photo: Emil Jeansson
Warm water from the Gulf Stream flows into the Nordic Seas. There the surface water cools, gets denser and mixes downward. In the Greenland Sea such mixing causes the formation of dense deep water.
Continuing southward in the straits between Greenland, Iceland, the Faroe Islands and Scotland, the dense deep water plunges like an underwater cascade to the bottom of the Atlantic Ocean.
The deep water formation in the Greenland Sea is crucial, not only locally, but for the whole system of ocean currents in the Atlantic.
"It is important to know how long time it takes the water to travel from point A to point B," says Emil Jeansson.
If you can find out how long ago the water left the surface, you will also know how fast it sinks.
Measures CFC in the deep ocean
The steel frame on the bench is about a meter long in each direction. Inside the frame, wires, tubes and rainbow-colored cables twist through glass flasks and a box with frost on the outside and seventy degrees Celsius below zero inside.
"A jungle," says Emil Jeansson.
A jungle and a jumble, yet a systematic assembly line. Water samples are fed in, and after the water has passed through all the tubes and containers – as well as an instrument standing beside – the results pop up as numbers and curves on a computer screen:
The water contains this much CFC. That means it is so many years old.
In addition to CFC-12 – freon – the instruments registers SF6, a compound used in industries such as aluminum production. Unlike CFCs, SF6 emissions continue to rise.
"SF6 og KFK-12 have a well-known history in the atmosphere," says Emil Jeansson.
Together these two substances provide a reliable reference to determine which year the water was last at the surface.
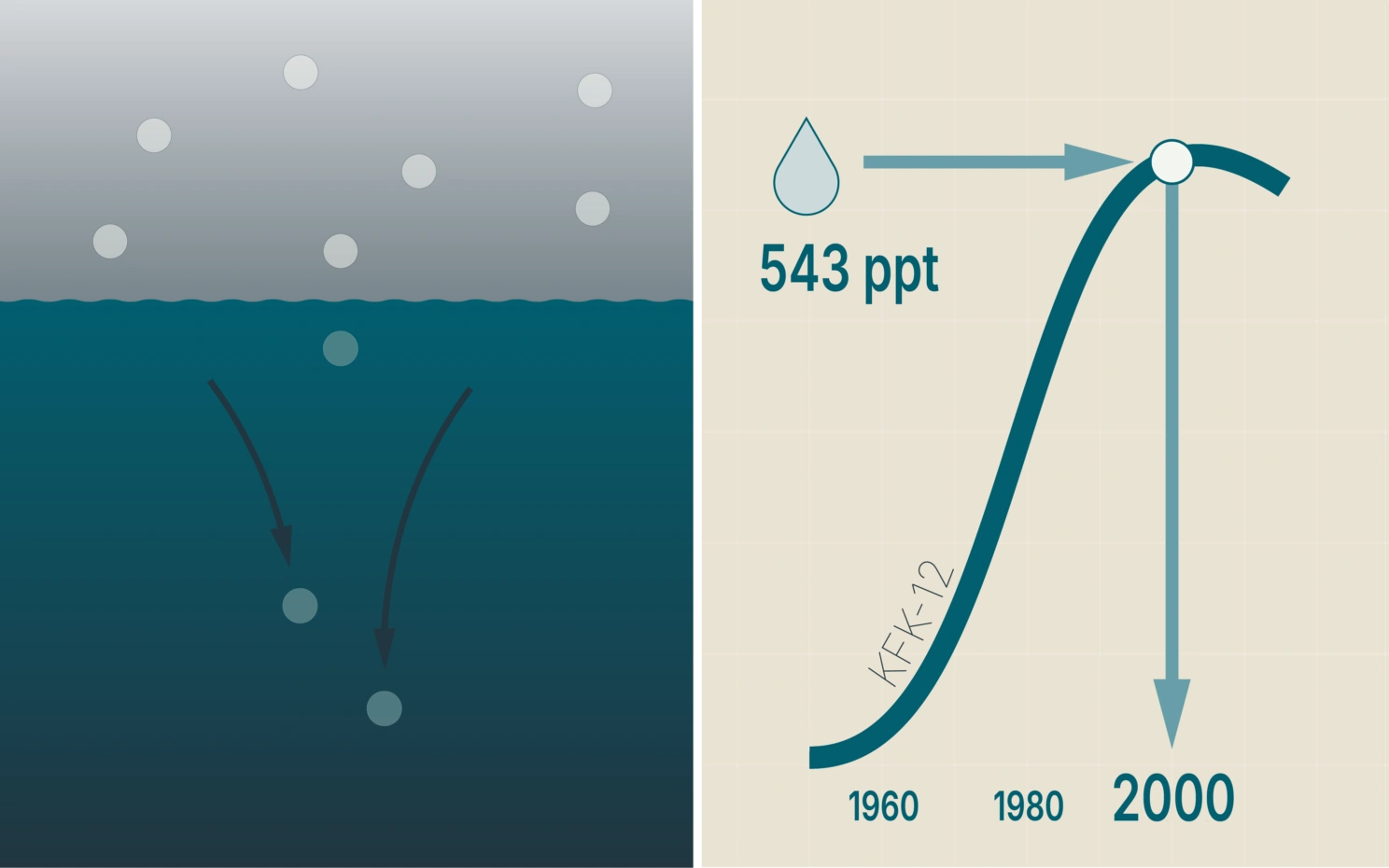
Sea water absorbs CFCs and other gases from the air. Knowing the CFC-12 content in sea water from a certain depth, you can calculate how much CFC-12 the atmosphere contained when this water was last at the surface. Comparing this with the development in the atmosphere from year to year, reveals the time of sinking. The year 2000, when the atmosphere contained 543 parts per trillion CFC-12, is used as an example. For improved accuracy and to distinguish between years with the same CFC-12 concentration, corresponding data for SF6 are added. The SF6 content in the atmosphere is still increasing. Ill.: Ellen Viste
Age tells of deep water formation
Water samples are collected from the sea outside, some not many meters below the hull of Kronprins Haakon, others at several thousand meters' depth.
When the sampled water was at the surface, it took up CFC from the air – substantial amounts in years with a lot of CFC in the atmosphere, less in other years. When the water was mixed downward, CFC and other gases were brought along. As a result, you may compare the concentration of CFC in a water sample with the development in the atmosphere.
Then you know when water began to sink.
"It may have been ten, fifty or a hundred years ago," says Emil Jeansson.
The age of the water indicates how fast the water reaches great depths and how extensive the sinking and formation of deep water in the Greenland Sea is. Combined with other measurements, these gases may also be used to calculate how much anthropogenic CO2 the deep water contains – a measure of how much CO2 the ocean absorbs from the atmosphere.

Emil Jeansson does not expect nature to be free of CFCs anytime soon. Photo: Ellen Viste
No shortage of CFCs
Almost forty years have passed since nations started phasing out CFCs. The CFC concentration in the atmosphere is declining. Will there be a time when there is not sufficient CFC-12 to trace sinking sea water?
"It is possible that other tracers may be relevant in the future," says Emil Jeansson.
The oceanographer dismisses the idea that a lack of CFCs will pose any problems. For now, SF6 concentration still rises, and CFC-12 is present at concentrations high enough to be easily detected.
"I will be able to measure CFCs for as long as I live," he says.
Related Projects

Resilient northern overturning in a warming climate
In ROVER the researchers will observe the deep-water formation along east Greenland, both through a winter expedition on an icebreaker, using mooring across the Greenland slope, and autonomous underwater gliders. The measurement campaign will provide groundbreaking data, cruical for the understanding of the processes that supply cold, deep water to the lower limb of the AMOC.